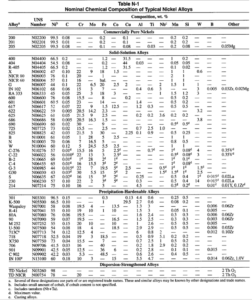
Nickel alloys offer unique physical and mechanical properties and are useful in a variety of industrial applications, notably because of their resistance to attack in various corrosive media at temperatures from 200°C (400°F) to over 1090°C (2000″F), and their good low- and high-temperature mechanical strength. In demanding industrial environments, nickel alloy welds must duplicate the attributes of the base metal to a very high degree. Welding, heat treating, and fabrication procedures should be established with this in mind. The chemical compositions of various nickel alloys are listed in Table N- 1.
High-quality weldments are readily produced in nickel alloys by commonly used welding processes. Not all processes are applicable to every alloy; metallurgical characteristics or the unavailability of matching or suitable welding filler metals and fluxes may limit the choice of welding processes.
Welding procedures for nickel alloys are similar to those used for stainless steel, except the molten weld metal is more sluggish, requiring more accurate weld metal placement in the joint. Thermal expansion characteristics of nickel alloys approximate those of carbon steel and are more favorable than those of stainless steel. Thus, warping and distortion are not severe during welding.
The mechanical properties of nickel alloy base metals will vary depending on the amount of hot or cold work remaining in the finished form (sheet, plate, or tube). Some modification in the procedures may be needed if the base metal is not in the fully annealed condition.
In general, the properties of welded joints in fully annealed nickel alloys are comparable to those of the base metals. Postweld treatment is generally not needed to maintain or restore corrosion resistance in most nickel alloys. In most media, the corrosion resistance of the weld metal is similar to that of the base metal. Welds made on Ni-Mo alloy NlOOOl and Ni-Si cast alloys commonly are solution annealed after welding to restore corrosion resistance to the heat-affected zone (HAZ).
Over-alloyed filler metals are often used (sometimes in lieu of postweld heat treatment) to fabricate components for very aggressive corrosive environments. The over-matching composition offsets the effects of weld metal segregation when using a matching composition. Examples are the use of filler metal NiCrMo-3 products to weld the “super” stainless alloys, containing 4 to 28% molybdenum, and the use of filler metal NiCrMo-10 to fabricate components of the base metal Ni-Cr-Mo alloy C-276 (UNS N10276).
Postweld heat treatment may be required for precipitation hardening in specific alloys. Postweld stress relief may be necessary to avoid stress-corrosion cracking in applications involving hydrofluoric acid vapor or certain caustic solutions. For example, Ni-Cu alloy 400 (UNS N04400) immersed in hydrofluoric acid is not sensitive to stress-corrosion cracking, but it is when exposed to the aerated acid or the acid vapors.
The choice of welding process will be based on the following:
(1) Alloy to be welded
(2) Thickness of the base metal
(3) Design conditions of the structure (such as temperature, pressure, or type of stresses)
(4) Welding position
(5)Need for jigs and fixtures
(6) Service conditions and environments
Metal Characteristics
Nickel has a face-centered-cubic (FCC) structure up to its melting point. Nickel can be alloyed with a number of elements without forming detrimental phases.
Nickel in some aspects bears a marked similarity to iron, its close neighbor in the periodic table. Nickel is only slightly denser than iron, and it has similar magnetic and mechanical properties. The crystalline structure of pure nickel at room temperature, however, is quite different from that of iron. Therefore, the metallurgy of nickel and nickel alloys differs from that of iron alloys.
Alloy Groups
Nickel alloys can be classified into four groups:
(1) Solid-solution-strengthened alloys
(2) Precipitation-hardened alloys
(3) Dispersion-strengthened alloys
(4) Cast alloys
Solid-Solution-Strengthened Alloys
All nickel alloys are strengthened by solid solution. Additions of aluminum, chromium, cobalt, copper, iron, molybdenum, titanium, tungsten, and vanadium contribute to solid-solution strengthening. Aluminum, chromium, molybdenum, and tungsten contribute strongly to solid-solution strengthening while others have a lesser effect. Molybdenum and tungsten improve strength at elevated temperatures.
Pure Nickel. Nickel 200 and the low-carbon version, nickel 201, are most widely used where welding is involved. Of these, the low-carbon nickel (201) is preferred for applications involving service exposure to temperatures above 315°C (600°F) because of its increased resistance to graphitization at elevated temperatures. This graphitization is the result of excess carbon being precipitated intergranularly in the temperature range of 315 to 760°C (600 to 1400’F) when nickel 200 is held there for extended time.
Major applications for the two alloys are food processing equipment, caustic handling equipment, laboratory crucibles, chemical shipping drums, and electrical and electronic parts.
Nickel-Copper Alloys. Nickel and copper form a continuous series of solid solutions with a face-centered-cubic crystal structure. The principal alloys in this group are alloy 400 and the free-machining version of it, R-405. These alloys have high strength and toughness, and they are important in industry primarily because of their corrosion resistance. The alloys have excellent resistance to sea or brackish water, chlorinated solvents, glass etching agents, sulfuric acids, and many other acids and alkalis.
Nickel-copper alloys are readily joined by welding, brazing, and soldering with proper precautions. To improve strength and to eliminate porosity in the weld metal, filler metals that differ somewhat in chemical composition from the base metal may be used. Welding without the addition of filler metal is not recommended for manual gas tungsten arc welding. Most automatic or mechanized welding procedures require the addition of filler metal, but a few do not.
Welding filler metals applicable to this alloy group are also widely used to weld copper alloys.
Nickel-Chromium Alloys. Nickel alloys 600, 601, 690,214, 230, G-30, and RA-330 are commonly used. Alloy 600, which is the most widely used, has good corrosion resistance at elevated temperatures along with good high-temperature strength. Because of its resistance to chloride-ion stress-corrosion cracking, it finds wide use at all temperatures and has excellent room-temperature and cryogenic properties.
Precipitation-HardenableAlloys
These alloys are strengthened by controlled heating, which precipitates a second phase known as gamma prime, from a supersaturated solution. Precipitation occurs upon reheating a solution-treated and quenched alloy to an appropriate temperature for a specified time. Each alloy will have an optimum thermal cycle to achieve maximum strength in the finished aged condition. Some cast alloys will age directly as the solidified casting cools in the mold.
The most important phase from a strengthening standpoint is the ordered face-centered-cubic gamma prime that is based upon the compound Ni3A1. This phase has a high solubility for titanium and niobium; consequently, its composition will vary with the base-metal composition and temperature of formation. Aluminum has the greatest hardening potential, but this is moderated by titanium and niobium. Niobium has the
greatest effect on decreasing the aging rate and improves weldability.
Nickel-Copper Alloys. The principal alloy in this group is K-500. Strict attention to heat-treating procedures must be followed to avoid strain-age cracking. Its corrosion resistance is similar to the solid-solution alloy 400. The alloy has been in commercial existence
for well over 50 years and is routinely welded, using proper care, with the gas tungsten arc welding process. Weld metal properties using filler metals of matching composition seldom develop 100% joint efficiencies, thus a common consideration by the designer is to locate the weld in an area of low stress. ERNiFeCr-2 filler metal has been used to join this alloy, but an evaluation of service environment and the differing aging temperatures between the two alloys must be made. The base metal supplier should be consulted for
recommendations for filler materials.
Dispersion-Strengthened Alloy
Nickel and nickel-chromium alloys can be strengthened to very high strength levels by the uniform dispersion of very fine refractory oxide (Tho2) particles
throughout the alloy matrix. This is done using powder metallurgy techniques during manufacture of the alloy. When these metals are fusion welded, the oxide particles agglomerate during solidification. This destroys the original strengthening afforded by dispersion within the matrix. The weld metal will be significantly weaker than the base metal. The high strength of these base metals can be retained with processes that do not
involve melting the base metal. Contact the base metal supplier for recommendations for specific conditions.
Cast Alloys
Casting alloys, like wrought alloys, can be strengthened by solid-solution or precipitation hardening. Precipitation-hardening alloys high in aluminum content, such as alloy 713C, will harden during slow cooling in the mold and are considered unweldable by fusion processes. However, surface defects and service damage are frequently repaired by welding. It should be understood that a compromise is being made between the convenience of welding and the cast strength and ductility. Most nickel cast alloys will contain significant amounts of silicon to improve fluidity and castability. Most of these cast alloys are weldable by conventional means, but as the silicon content increases, so does weld-cracking sensitivity. This cracking sensitivity can be avoided using welding
techniques that minimize base metal dilution.
Nickel castings that are considered unweldable by arc welding methods may be welded using the oxyacetylene process and a very high preheat temperature. Cast nickel alloys containing 30% copper are considered unweldable when the silicon exceeds 2% because of their sensitivity to cracking. However, when weldable grade castings are specified, weldability is quite good, and such welds will pass routine weld-metal inspections using methods such as radiography, liquid penetrant testing, and pressure tests.
