Metallurgy is defined as the science and technology of metals, and consists of two broad divisions:
(1) Process metallurgy, which involves the reduction of ores, refining of metals, alloying, casting, and the working and shaping of metal into semifinished and finished products
(2) Physical metallurgy, which includes heat treatment, mechanical testing, metallography and other subjects dealing with the application, design, testing, and inspection of metal products.
Both process metallurgy and physical metallurgy are involved in welding. Welding can be compared to a series of metallurgical operations involved in metal production, like steel making, but welding is performed on a small scale with the pertinent steps carried out in rapid succession. During most welding processes, a volume of molten metal (weld pool) is formed (cast) within the confines of solid base metal
(mold). Weld metal initiates solidification in a unique manner, unlike molten metal cast in a conventional mold. Weld metal is susceptible to blowholes and internal porosity caused by the evolution of gases, as experienced in ingot making and castings.
The base metal of a weld can be preheated to retard the cooling rate and solidification, just as preheated molds are used to retard the solidification of castings.
The striking difference between welding and other metal-producing operations is the contrast in the mass of metal involved and the effect of mass on physical and metallurgical changes. Welding involves comparatively small masses that are heated very rapidly by intense heat sources and that cool rapidly because of intimate contact with a larger surrounding mass of colder base metal. Consequently, it can be expected that weld zones are prone to display unusual structures and properties.
Welding involves many metallurgical phenomena, such as melting, freezing, solid state transformations, thermal strains and shrinkage stresses that can cause many practical problems. These problems can be avoided or solved by applying appropriate metallurgical principles to the welding process.
An understanding of welding metallurgy requires a broad knowledge of general metallurgy. For this reason, general metallurgy is addressed first, followed by specific aspects of welding metallurgy. The brief description of general metallurgy is only an outline of topics necessary to provide a basis for welding metallurgy. For a more complete treatment of metallurgy the reader should refer to the specific references at the end of this article.
General Metallurgy
Structure of Metals. Solid metals have a crystalline structure in which the atoms of each crystal are arranged in a specific geometric pattern. This orderly arrangement of the atoms, called a lattice, is responsible for many of the properties of metals. The most
common lattice structures found in metals are listed in Table M- 13. Their atomic arrangements are illustrated in Figure M-5.
Each grain in a pure metal at any particular temperature has the same crystalline structure and the same atomic spacing as all of the other grains. However, each grain grows independently of every other grain, and the orientation of the grain lattice differs from one grain to another. The periodic and orderly arrangement of the atoms is disrupted where the grains meet, and the grain boundaries form a continuous network throughout the metal. Because of this grain boundary disorder, differences in the behavior of the metal often occur at those locations.
Up to this point, only pure metals have been considered. However, most common engineering metals contain residual or intentionally added metallic and nonmetallic elements dissolved in the matrix.

These ingredients, called alloying elements, affect the properties of the base metal. The atomic arrangement (crystal structure), the chemical composition, and the thermal and mechanical history have an influence on the properties of an alloy.
Alloying elements, called solutes, are located in the parent metal matrix in one of two ways. The solute atoms may occupy lattice sites replacing some of the parent metal atoms, called solvent. Alternatively, if the solute atoms are small enough, they may fit into the spaces between the solvent atoms.
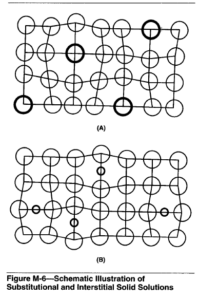
Substitutional Alloying. If the solute atoms are similar in size and chemical behavior to the solvent atoms, they may occupy sites at the lattice locations as shown in Figure M-6 (A). This type solid solution is called substitutional. Examples of substitutional solid solutions are gold dissolved in silver, or copper dissolved in nickel.
Interstitial Alloying. When the alloying atoms are very small in relation to the parent atoms, they can locate (or dissolve) in the spaces between the parent metal atoms without occupying lattice sites. This type of solid solution is called interstitial, and is illustrated
in Figure M-6 (B). Small amounts of carbon, nitrogen, or hydrogen for example, alloy interstitially in iron and other metals.
Multiphase Alloys. Frequently, the alloying atoms cannot dissolve completely, either interstitially or substitutionally. The result, in such cases, is the formation of mixed atomic groupings (different crystalline structures) within a single alloy. Each different crystalline structure is referred to as a phase, and the alloy is called a multiphase alloy. The individual phases may be distinguished from one another, under a microscope at magnifications of 50X to 2000X, when the alloy is appropriately polished and etched.
All commercial metals consist of the primary or basic element and smaller amounts of one or more alloying elements. The alloying elements may be intentionally added or may be residual (tramp) elements. Commercial metals can be single or multiphase alloys. Each phase will have its own characteristic crystalline structure.
The overall arrangement of the grains, grain boundaries, and phases present in a metal alloy is called the microstructure of the alloy. The microstructure is largely responsible for the physical and mechanical properties of the metal. It is affected by both the chemical composition and the thermal and mechanical history of the metal.
Consequently, microstructure is also affected by welding, but the effect is confined to the local region of the weld. The metallurgical changes in the local region (called the heat-affected zone) can have a profound effect on the service performance of a
Fine-grained materials generally have better mechanical properties for service at room and low temperatures. Conversely, coarse-grained materials generally perform better at high temperatures.
Phase Transformations
Critical Temperatures. At specific temperatures, the atoms of many metals change their crystallographic structure. For example, the crystalline structure of pure iron at temperatures up to 910°C (1670°F) is body-centered cubic, Figure M-5 (B). From 910 to
1390°C (1670 to 2535oF), the structure is face centered cubic, Figure M-5 (A), and from 1390 to 1535°C (2535 to 2795oF), the melting temperature, it is again body centered cubic. A phase change in crystal structure in the solid state is known as an allotropic
transformation. Other metals that undergo allotropic transformations are titanium, zirconium, and cobalt. Factors that influence the temperature at which transformation
takes place are chemical composition, cooling rate, and the presence of stress.
A metal also undergoes a phase change when it melts or solidifies. Pure metals melt and solidify at a single temperature. Alloys, on the other hand, usually melt and solidify over a range of temperatures. The exception to this rule is the eutectic composition.
Phase Diagrams. Metallurgical events, such as phase changes and solidification, are best illustrated with a drawing called a phase diagram (sometimes referred to as an equilibrium diagram or a constitution diagram).
Phase diagrams only approximately describe commercial alloys because most published phase diagrams are based on two-component systems at equilibrium. Most commercial alloys have more than one component, and equilibrium conditions are approached only at high temperatures. However, with a knowledge of the normal responses of the alloy, a phase diagram is a powerful tool in understanding the behavior of commercial metals.
Phase diagrams for systems with more than two components are complex and more difficult to interpret, but still provide the best way to study most alloy systems.
Effects of Deformation and Heat Treatment
Deformation and Annealing of Metals. When metals are plastically deformed at room temperature, a number of changes takes place in the microstructure. Each individual grain must change shape to produce the anticipated overall deformation. As deformation proceeds, each grain becomes stronger and, therefore, more difficult to deform further. This behavior is called work hardening. When the metal is deformed below a critical temperature, there is a gradual increase in the hardness and strength of the metal and
a decrease in ductility. This phenomenon is known as cold working.
If the metal is worked moderately or severely and then heated to progressively higher temperatures, several things happen. At temperatures up to about 205°C
(400°F) there is a steady decline in the residual stress level, but there is virtually no change in microstructure or properties. At about 205 to 230°C (400 to 450oF), a
relatively low level of residual stress remains, but the microstructure has not changed. The strength of the metal remains relatively unchanged compared to that of the original cold-worked material, and the ductility, while improved, is still rather low. This reduction in stress level and the improvement in ductility are attributed to the metallurgical phenomenon called recovery, a term indicating a reduction in crystalline stresses without accompanying microstructural changes.
When the cold-worked metal is heated to a temperature above 230°C (450″F), mechanical property changes become apparent, as do changes in microstructure. In place of the deformed grains, a new group of grains form and grow. These grains replace the old grains, and eventually all signs of the deformed grains disappear. The new microstructure resembles the original microstructure (before cold-working), and the
metal is softened and made more ductile than it was in the cold-worked condition. This process is called recrystallization, a necessary part of annealing procedures. (Annealing refers to a heating and cooling process usually applied to induce softening). When heated
to higher temperatures, the grains begin to grow and the hardness and strength of the metal are significantly reduced. Metals are often annealed prior to further cold working or machining.
Metallurgy of Welding
A welded joint consists of weld metal (which has been melted), heat-affected zones, and unaffected base metals. The metallurgy of each weld area is related to the base and weld metal compositions, the welding process, and the procedures used. Most typical weld metals have rapidly solidified, and usually have a fine grain dendritic microstructure. The weld metal is an admixture of melted base metal and deposited (filler) metal, if used. Some welds (autogenous) are composed of only remelted base metal. Examples of autogenous welds are gas tungsten arc and electron beam welds made without filler metal, and resistance welds. In most arc welding processes, a filler metal is used.
To achieve mechanical and physical properties that nearly match those of the base metal, a filler metal is often selected which is similar in chemical composition to the base metal. This is not a universal rule; sometimes the weld metal composition is deliberately
made significantly different from that of the base metal. The intent is to produce a weld metal with properties compatible with the base metal. Therefore, variations from the base metal composition are not uncommon in filler metals.
When a weld is deposited, the first grains to solidify are nucleated by the unmelted base metal, and these grains maintain the same crystal orientation. Depending on composition and solidification rates, the weld solidifies in a cellular or a dendritic growth mode. Both modes cause segregation of alloying elements. Consequently, the weld metal may be less homogeneous than the base metal.
The weld heat-affected zone is adjacent to the weld metal. The heat-affected zone is that portion of the base metal that has not been melted, but whose mechanical properties or microstructure have been altered by the heat of welding. The width of the heat- affected zone is a function of the heat input. The heat-affected zone may in theory include all regions heated to any temperature above the ambient. From a practical viewpoint, however, it includes those regions which are actually influenced by the heat of the welding process.
For a plain carbon as-rolled steel, the heat of welding has little influence on those regions heated to lessthan about 700°C (1350°F). For a heat-treated steel that was quenched to martensite and tempered at 3 15°C (600″F), heating above this temperature would change the mechanical properties of the metal. For a heat-treated aluminum alloy age hardened at 120°C (250oF), any portion of a welded joint heated above this temperature is the heat-affected zone.
Heat-affected zones are often defined by the response of the welded joint to hardness variation or microstructural changes. Thus, changes in microstructure produced by the welding heat which are seen in etching or in hardness profiles may be used to establish the heat-affected zone. In many cases, these are arbitrary measures of the heat-affected zone, although they may be of practical value in testing and evaluating welded joints.
Adjacent to the heat-affected zone is the unaffected base metal. The base metal is selected by the designer for the specific application based on a specific property or combination of properties, such as yield or tensile strength, notch toughness, corrosion resistance, or density. It is the job of the welding engineer to select the welding consumables and process to develop welding procedures that allow the design properties to be fully utilized in service. The characteristic of a metal that allows it to be welded without losing its desirable properties is called weldability.
Weld Metal. The microstructure of the weld metal is considerably different from that of the base metal. The difference in microstructure is not related to chemical compositions, but to different thermal and mechanical histories of the base metal and the weld metal. The structure of the base metal is a result of a hot rolling operation and multiple recrystallization of the hot-worked metal. In contrast, the weld metal has not been mechanically deformed and therefore, has an as-solidified dendritic structure. This structure and its attendant mechanical properties are a direct result of the sequence of events that occur as the weld metal solidifies. These events include reactions of the weld metal with gases in the vicinity of the weld and with nonmetallic liquid phases (slag or flux) during welding, and also reactions that took place in the weld after solidification.
Solidification.The unmelted portions of grains in the heat-affected zone at the solid-liquid interface serve as nucleation sites for weld metal solidification. Metals grow more rapidly in certain crystallographic directions. Therefore, favorably oriented grains grow for
substantial distances, while the growth of others that are less favorably oriented is blocked by other grains.
As a result, weld metal often exhibits a microstructure, described as columnar, in which the grains are relatively long and parallel to the direction of heat flow. This structure is a natural result of the influence of favorable crystal orientation on the competitive nature of solidification grain growth.
Dendrites. Weld metal solidification of most commercial metals involves micro-segregation of alloying and residual elements. This action is associated with, and, in large measure, responsible for the formation of dendrites. A dendrite is a structural feature which reflects the complex shape taken by the liquid-solid interface during solidification.
As the primary dendrites solidify, solutes that are more soluble in the liquid are rejected by the solid material and diffuse into the remaining liquid, lowering the freezing point. As the solute alloys concentrate near the solid-liquid interface, crystal growth is
arrested in that direction. The grains then grow laterally, producing the dendrite arms characteristic of as-solidified metals. Many dendrites may grow simultaneously into the liquid from a single grain during solidification. Therefore, each of these dendrites has
the same crystal orientation, and they will all be part of the same grain. However, a solute-rich network will exist among the dendrites in the final structure.
The general tendency is for weld-metal grain size to increase with heat input, but there is no fixed relationship. The grain size may be influenced by nucleating agents, vibration, or other process variables, but the dendrite arm spacing is exclusively a function of solidification rate which is controlled by heat input.
Gas Metal Reactions
Gas-metal reactions depend on the presence of oxygen, hydrogen, or nitrogen used alone or combined, in the shielding atmosphere. There are many sources for
these elements. Oxygen is intentionally added to argon in gas metal arc welding of steel to stabilize the arc. It can also be drawn in from the atmosphere or result from the dissociation of water vapor, carbon dioxide, or a metal oxide. Air is the most common source of nitrogen, but there are many sources of hydrogen, principally from atmospheric moisture, moisture in electrode coatings, slag, and shielding gases. Hydrogen may be present in solid solution in nonferrous metals or in surface oxides and lubricating compounds from the wire drawing operation.
Welding Ferrous Metals. Gas-metal reactions in welding steels occur in several steps. First, the gas molecules are broken down in the high temperature of the welding atmosphere and then the gas atoms dissolve in the liquid metal. Oxygen and nitrogen will
generally react with intentionally added deoxidizers such as manganese, silicon, and aluminum. These oxides will form a slag and float to the surface of the weld or precipitate as discreet oxides. Oxides and nitrides are present as small discreet particles.
Although they reduce the ductility and notch toughness of steel weld metal, the resulting mechanical properties are satisfactory for most commercial applications.
In consumable electrode welding, the oxide content of steel weld metal is significantly greater than the nitrogen content because oxygen is intentionally
present in arc atmospheres, whereas nitrogen is not. If the weld metal does not contain sufficient deoxidizers, the soluble oxygen will react with soluble carbon to produce CO or C02 during solidification. The gas molecules will be rejected during solidification and
produce porosity in the weld metal.
Hydrogen is always present in the arc atmosphere, if only in small quantities. Hydrogen atoms are soluble in liquid steel and less soluble in solid steel. Excess hydrogen that is rejected during solidification will cause porosity. A more significant problem is created by the hydrogen that remains dissolved in the solid steel.
Welding Nonferrous Metals. The primary gas-metal reactions of concern are the solution, reaction, and evolution of hydrogen or water vapor. These gases, therefore, should be excluded from the molten weld pool. With aluminum and magnesium, hydrogen is often introduced into the weld pool from hydrated oxides on the surfaces of the filler wire or workpieces, or both. It is rejected from the metal during solidification to produce porosity. For this reason, cleaned aluminum and magnesium filler metals should be stored in sealed, desiccated containers. Mechanical cleaning or vacuum heating at 150°C (300°F) is recommended for workpieces or filler metals which have been exposed to moist air. The hydrogen solubility difference between the liquid and solid states for magnesium is less than that for aluminum. Consequently, the
tendency for hydrogen-produced porosity is lower in magnesium
In the case of copper and copper alloys, hydrogen will react with any oxygen in the molten weld pool to produce water vapor, and thus porosity, during solidification. The filler metals for copper alloys contain deoxidizers to prevent this reaction. Porosity caused by water vapor will not form in alloys of zinc, aluminum, or beryllium because these elements form stable oxides. Porosity from water vapor can form in nickel-copper and nickel alloy weld metal, and filler metals for these alloys should contain strong deoxidizers.
Titanium alloys are embrittled by reaction with a number of gases including nitrogen, hydrogen, and oxygen. Consequently, these elements should be excluded from the arc atmosphere. Welding should be done using carefully designed inert gas shielding or in a vacuum. Titanium heat-affected zones are also significantly embrittled by reaction with oxygen and nitrogen. Titanium weldments should be shielded so that any surface heated to over 260°C (500°F) is completely protected by an inert gas. Hydrogen is the
major cause of porosity in titanium welds. The hydrogen source, as in other nonferrous and ferrous metals, can be the filler metal surface. In addition, soluble hydrogen in the filler metal and the base metal can contribute significantly to the total hydrogen in the
Liquid Metal Reactions. During the welding process, nonmetallic liquid phases that interact with the molten weld metal are frequently produced. These liquid phases are usually slag formed by the melting of an intentionally added flux.
The slags produced in the shielded metal arc welding (SMAW), submerged arc welding (SAW), and electroslag welding (ESW) processes are designed to absorb deoxidation products and other contaminants produced in the arc and molten weld metal. The quantity and type of nonmetallic deoxidation products generated when arc welding steel are primarily silicates of aluminum, manganese, and iron, that float to the surface of the molten weld pool and become incorporated in the slag. Some products can be trapped in the weld metal as inclusions.
Hot Cracking. Another important effect that results from the interaction of the liquid and solid state is the weld defect referred to as hot cracking. Shrinkage stresses produced during solidification become concentrated in a small liquid region and produce microcracks between the dendrites. These cracks are called hot cracks because they occur at temperatures close to the solidification temperature.
The most common cause of hot cracking is the presence of low-melting alloy sulfides that wet the dendrite surfaces. In some ferrous alloys, such as stainless steels, silicates have also been found to produce cracking. Avoidance of cracking in these alloys is usually accomplished by controlling both the amount and type of sulfides that form and the minor alloy constituents that may promote cracking.
Solid State Reactions. In terms of the behavior of weld metals, there are a number of solid state reactions that are important as strengthening mechanisms in the weld metal itself. There are some important phenomena involving solid state transformations and subsequent reactions with dissolved gases in the metal. The most significant of these phenomena is the formation of cold cracks in steel weld metal or heat-affected zones, often referred to as delayed cracking. The steels most susceptible to this type of cracking are those that can transform to martensite on cooling from the weld thermal cycle. The cracking occurs after the weld has cooled to ambient temperature, sometimes hours or
even days after welding. It is always associated with dissolved hydrogen in the weld metal which remains there during solidification and subsequent transformation to martensite.
Because delayed cracking is always associated with dissolved hydrogen, two precautions are universally used to minimize the risk of delayed cracking. They
are:
(1) Preheating the base metal to slow the cooling rate.
(2)Using low-hydrogen welding processes.
The use of preheat prevents the formation of a crack susceptible microstructure and also promotes the escape of hydrogen from the steel by diffusion.
Hydrogen is relatively soluble in austenite, and virtually insoluble in ferrite. On rapid cooling, the austenite transforms either to an aggregate of ferrite and carbide or to martensite, and hydrogen is trapped in solution. In a plain carbon steel, this transformation takes place at a relatively high temperature, even if cooling is rapid, and the hydrogen atoms have sufficient mobility to diffuse out of the metal. A rapidly cooled hardenable steel transforms at a much lower temperature where the hydrogen atoms have lower mobility, the microstructure is martensitic, and crack sensitive, and this combination will likely cause cracking. The association of hydrogen with delayed cracking led to the development of low-hydrogen covered electrodes. Low-hydrogen electrode coverings must be kept essentially moisture free since moisture is a primary source of hydrogen.
Another solid state reaction that affects weld joint mechanical properties in ferrous and nonferrous alloys is the precipitation of second phases during cooling.
Precipitation of a second phase in grain boundaries is particularly deleterious because the grain boundaries are continuous throughout the metal. A concentration of a second phase at grain boundaries may significantly reduce ductility and toughness.
Strengthening Mechanisms in Weld Metal
The practical methods for strengthening weld metals are fewer than for base metals. For example, weld metal is not usually cold worked. There are four mechanisms for strengthening weld metal, and where applicable they are additive: (1) solidification grain structure, (2) solid solution strengthening, (3)transformation hardening, and (4)precipitation hardening.
The first mechanism is common to all welds, the second is applicable to any alloy type, but the third and fourth apply only to specific types of alloys.
Solidification Grain Structure. Weld metal freezes rapidly, creating a segregation pattern within each grain. The resulting microstructure consists of fine dendrite arms in a solute-rich network. This type of microstructure impedes plastic flow during tensile testing. As a result, weld metals typically have a higher yield-to-tensile strength ratio than base metals.
Solid Solution Strengthening. Weld metal is strengthened by alloying elements present. Both substitutional and interstitial alloying elements will strengthen ferrous and nonferrous weld metal.
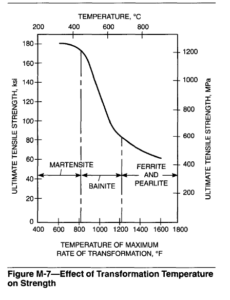
Transformation Hardening. Hardening will result in ferrous weld metal even if the austenite decomposition product is not martensite. The rapid cooling rates, achieved during the cooling portion of weld thermal cycles, decrease the austenite transformation temperature. The ferrite-carbide aggregate formed at low transformation temperatures is finer and stronger than that formed at higher transformation temperatures. The effect of transformation temperature on the ultimate tensile strength of steel weld metal is shown in Figure M-7.
Precipitation Hardening. Weld metal of precipitation hardening alloy systems can be strengthened by an aging process. In most commercial applications, the precipitation hardened weldments are aged after welding without the benefit of a solution heat treatment. In multipass welds, some of the zones of weld metal will be aged or overaged from the welding heat. The heat-affected zone will also contain overaged metal. An
aging heat treatment will strengthen the weld metal and the heat-affected zone. The weld metal and the heat-affected zone may not strengthen to the same level as the base metal due to the presence of overaged metal. Some aluminum precipitation hardening weld
metals will age naturally at room temperature.
The Heat-Affected Zone
The strengthened toughness of the heat-affected zone in a welded joint is dependent on the base metal, the welding process, and the welding procedure.
Because the weld thermal cycle is generally a rapid one, the base metals most influenced by welding will be those strengthened or annealed by heat treatments. The temperatures in the weld heat-affected zone vary from ambient to near the liquidus temperature. Metallurgical processes that proceed slowly at lower temperatures can proceed rapidly to completion at temperatures close to the liquidus.
To understand the various effects of welding heat on the heat-affected zone, these effects can basically be considered in terms of four different types of alloys that can be welded. Some alloys can be strengthened by more than one of these processes, but for simplicity the processes are considered separately.
Solid-Solution Strengthened Alloys. Solid-solution alloys normally exhibit the fewest weld heat-affected zone problems. If they do not undergo a solid state transformation, the effect of the thermal cycle is small, and the properties of the heat-affected zone will be
largely unaffected by welding. Grain growth will occur next to the fusion line as a result of the high peak temperature. This will not significantly affect mechanical properties if the grain-coarsened zone is only a few grains wide.
Commonly used alloys strengthened by solid solution are annealed aluminum alloys, annealed copper alloys, and hot rolled and annealed low-carbon steels. Annealed ferritic and austenitic stainless steels come under essentially the same category.
Strain Hardened Base Metals. Strain hardened base metals will recrystallize when heated above the recrystallization temperature. The heat of welding will recrystallize the heat-affected zones in cold worked metals and soften the metal considerably. The recrystallized heat-affected zone is softer and weaker than the cold worked base metal, and the strength cannot be recovered by heat treatment.
If the cold worked materials undergo an allotropic transformation when heated, the effects of welding are even more complex. Steel and titanium alloys may have two recrystallized zones. The first fine-grained zone results from recrystallization of the cold worked alpha phase. The second fine-grained zone results from the allotropic transformation to the high temperature phase.
Precipitation-Hardened Alloys. Alloys that are strengthened by precipitation hardening respond to the heat of welding in the same manner as work hardened alloys; that is, the heat-affected zone undergoes an annealing cycle. The response of the heat-affected zone is more complex because the welding thermal cycle produces different effects in different regions. The heat treating sequence for precipitation hardening is: solution treat, quench, and age. The welding heat will re-solution treat the heat-affected zone regions closest to the weld, and produce a relatively soft single phase solid solution with some coarse grains. This region can be hardened by a post weld aging treatment.
Those regions of the heat-affected zone that are heated to temperatures below the solution treatment temperature will be overaged by the welding heat. A postweld aging treatment will not reharden this region. If the welding heat does not raise the heat affected
zone temperature above the original aging temperature, the mechanical properties are not significantly affected.
It is difficult to weld high-strength precipitation hardenable alloys without some loss of strength, but three techniques may be used to minimize the loss. The most effective of these techniques is to re-solution treat, quench, and age the weldment. This technique is expensive, and in many cases may not be practicable. A second approach would be to weld precipitation hardened base metal and then re-age the weldment.
This raises the strength of the solution-treated region of the heat-affected zone, but does not improve the strength of the overaged zone. Another alternative is to weld the base metal in the solution treated condition and age the completed weldment. The overaged zone is still the weakest link, but the overall effect may be an improvement over the previous approaches.
Since it is the weld thermal cycle that lowers the strength of the heat-treated base metal, high heat input welding processes are not recommended for precipitation-hardened alloys. Low heat input will minimize the width of the heat-affected zone and the amount of softened base metal.
Transformation Hardening Alloys. The transformation hardening alloys of interest are the steels with sufficient carbon and alloy content to transform to martensite upon cooling from welding. These may be steels which are already heat treated to tempered martensite
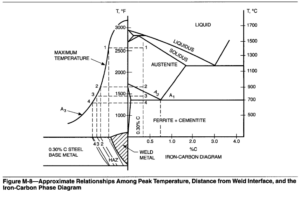
prior to welding, or steels that have adequate hardenability to transform to martensite during a weld thermal cycle, even though they may not have been heat treated. In either case, the heat-affected zone is affected by the weld thermal cycle in approximately the same manner. The heat-affected zones, together with the steel portion of the iron-carbon phase diagram, are illustrated in Figure M-8.
In Figure M-8, the grain coarsened region is near the weld interface (Region 1). Rapid austenitic grain growth takes place in this region when exposed to the near melting point temperatures. The large grain size increases hardenability, and this region can readily transform to martensite on cooling. Region 2 is austenitized, but the temperature is too low to promote grain growth. The hardenability of Region 2 will not be significantly increased by grain growth, but may still transform to martensite if the cooling rate is fast enough or if the alloy content is great enough. In Region 3, some grains transform to austenite and some do not. The austenite grains are very fine. No austenitic transformation takes place in Region 4 next to the unaffected base metal, but the ferrite grains may be tempered by the heat of welding.
The width of the heat-affected zone and the widths of each region in the heat-affected zone are controlled by the welding heat input. High heat inputs result in slow cooling rates, and therefore, the heat input may determine the final transformation products.
High-carbon martensite is hard and strong, and it can create problems in the heat-affected zone. The hardness of the weld heat-affected zone is a function of the base metal carbon content. The hardness and crack-susceptibility increase and the toughness decreases with increasing carbon content. Martensite alone will not cause cracking; dissolved hydrogen and residual stresses are also present.
The same precautions used to prevent delayed cracking in weld metal will also prevent cracking in the heat-affected zone. The hardness of a weld heat affected zone is usually a good indication of the amount of martensite present and the potential for cracking. Cracking rarely occurs when the weld hardness is 250 HB or less, but is common when the hardness approaches 450 HB and no precautions are taken.
Special precautions may be necessary when welding hardenable steels that have been intentionally heat treated to produce a tempered martensitic microstructure. It is usually desirable to use a low welding heat input to control the size of the heat-affected zone, and a high preheat temperature to control the cooling rate of the weld. The welding recommendations of the steel manufacturer should be followed in preparing welding procedures for low-alloy, high-strength steels.
Base Metal
The third component in a welded joint is the base metal. Many of the common engineering materials available today are readily weldable. However, some materials are more difficult to weld and require special precautions.
Weldability. Weldability is the capacity of a material to be welded into a specifically designed structure under the imposed fabrication conditions, and to perform satisfactorily in the intended service. Some systems may have poor weldability under certain conditions and have satisfactory weldability under other conditions. For example, all grades of ASTM A514 (a heat treated 690 MPa [l00 ksi] yield strength constructional alloy steel) have satisfactory weldability, provided the base metal is sufficiently reheated, a low-hydrogen welding process is followed, and the heat input limitations are not exceeded.
The primary factor affecting the weldability of a base metal is its chemical composition or the grade of the material. Each grade of material has welding procedural limits within which sound weldments with satisfactory properties can be fabricated. If these limits are wide, the grade is said to have good weldability. If the limits are narrow, the material is said to have poor weldability. If extraordinary precautions are necessary, then the material is often said to be “unweldable.” Yet, in some cases and in some industries, “unweldable” materials are routinely welded under tight controls with vigorous inspection procedures and acceptance criteria. These methods are followed because welding may be the only (or at least the best) method to achieve the desired function within the design criteria for the whole assembly.
