Cryogenics is the science of very low temperatures and their phenomena. The definition for cryogenic temperatures has changed during the years as it became easier to produce cryogenic products. In about 1950, the definition of a cryogenic temperature was -73°C (-100°F). Now it has been reduced to -128°C (-200°F). Carbon dioxide, which boils at -78°C (-109°F) is no longer considered a cryogenic gas.
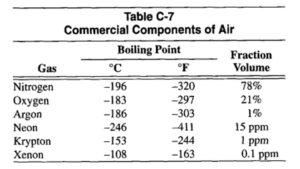
The commercial gas components in air, boiling temperatures at atmospheric pressure, and volume fraction in air are shown in Table C-7.
The welding industry relies heavily on cryogenic technology, because oxygen is needed for oxyfuel welding and cutting, and argon is needed for the GMAW and GTAW processes. These gases, along with nitrogen, are produced in air separation plants using a process developed by Dr. Karl von Linde about 1890. By 1910, air separation plants, although small, became relatively common in the United States.
Air separation plants have increased in size to the extent that a single plant can produce 2800 tons of oxygen per day. Air separation plants use high volume, low pressure pumps, turbo-expanders and reversing exchangers to drop the incoming air temperature below the -198°C (-325°F) needed to liquefy it. The liquid is then separated by fractional distillation in bubble towers; the liquid oxygen is removed at the bottom and the cooler nitrogen from the top. The separation and purification processes continue until the gases listed in Table C-7 are produced at the purity levels required by customers.
In addition to the welding industry, a diversity of industries depend heavily on cryogenic gases for their technology. For example, liquid oxygen and argon are supplied by the ton for refining steel and heat treating; liquid nitrogen is used for freezing foods.
