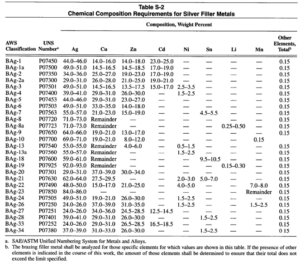
Silver-base filler metals (AWS Classification BAg) are used extensively in brazing both ferrous and non-ferrous metals and alloys, except aluminum and magnesium. This classification includes a range of silver based filler metal composition which may have various additions such as copper, zinc, cadmium, tin, manganese, nickel and lithium. Table S-2 lists chemical composition requirements for silver brazing filler metals.

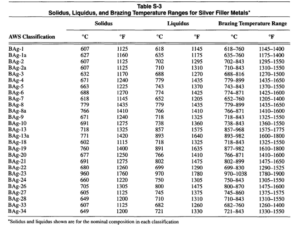
Silver brazing alloys are generally used in those cases where strength and resistance to shock are required. Examples of silver brazing applications are joining band saws, shrouds, and lacing wire for turbine blades, and in the fabrication of equipment where appearance as well as strength is important. Silver brazing alloys usually contain varying percentages of silver, copper and zinc, and are often called silver solders. These compositions have melting points from 700 to 870°C (1300 to 1600″F), depending on the proportions of the different metals, or a range below that of base metal brazing alloys or copper welding rods, which require from 870 to 1090°C (1600 to 2000°F). Table S-3 lists the brazing temperature ranges for the various silver brazing filler metals.
.png)
Selecting Silver Filler Metals
Silver, alloyed with copper in a proportion of 72% silver, 28% copper, forms a eutectic with a melting point of 780°C (435°F). This filler metal (BAg-8) can be used to furnace braze nonferrous base metals in a protective atmosphere. This alloy, however, does not easily wet ferrous metals. The addition of zinc lowers the melting temperature of the silver copper binary alloys and helps wet iron, cobalt, and nickel. Cadmium is also effective in lowering the brazing temperature of these alloys and assists in wetting a variety of base metals. Cadmium oxide present in brazing fume is poisonous, and cadmium-free filler metals should be utilized wherever possible. Tin can effectively reduce the brazing temperature, and is used to replace zinc or cadmium in filler metals. Nickel is added to assist in wetting tungsten carbides and provides greater corrosion resistance. Brazing alloys containing nickel are especially recommended for joining stainless steels because they reduce susceptibility to interfacial corrosion. Manganese is sometimes added to improve wetting on stainless steel, other nickel-chromium alloys, and cemented carbides. Lithium is effective in reducing oxides of refractory metals to promote filler metal wetting, and improve flow on stainless steels furnace brazed in protective atmospheres.
Flux is required when torch brazing with these filler metals in an oxidizing environment. Mineral fluxes conforming to AWS FB3A, or other classifications, in powder, paste, or slurry form are generally used. Vapor flux introduced through a torch flame also is suitable although filler metal capillary action may be limited with this type application. Vapor (gas) flux is normally used as a supplement to mineral flux types, to improve protection, wetting and flow.
Silver brazing filler metals are available in numerous forms including: wire, rod, pre-formed shapes, paste, powder, and strip. Several filler metals are available as a clad or “sandwich” strip with filler metal bonded to both sides of a copper core. This clad strip is popular in brazing carbide tool tips. The copper core absorbs stresses set up by differences in thermal expansion between the carbide and base metal, thus helping to prevent cracking.
BAg-1 brazing filler metal has the lowest brazing temperature range of the BAg filler metals. Because of this, it flows freely into tight capillary joints. Its narrow melting range is suitable for rapid or slow methods of heating. This filler metal also contains cadmium, and toxic fumes may be formed when it is heated. Precautions must be taken to assure proper ventilation of the brazing area to protect brazing personnel. BAg-la brazing filler metal has properties similar to BAg-1. Either composition may be used where low-temperature, free-flowing filler metals are desired. This filler metal also contains cadmium, and fume hazards must be eliminated.

BAg-2 brazing filler metal, like BAg-1, is freeflowing and suited for general purpose work. Its broader melting range is helpful where clearances are wide or not uniform. Unless heating is rapid, care must be taken that the lower melting constituents do not separate by liquation. This filler metal contains cadmium and fumes are toxic.
BAg-2a brazing filler metal is similar to BAg-2, but is more economical than BAg-2 since it contains 5% less silver. This filler metal contains cadmium, and fumes formed on heating are toxic.
BAg-3 brazing filler metal is a modification of BAg-la; i.e., nickel is added. It has good corrosion resistance in marine environments and caustic media and when used on stainless steel will inhibit crevice (interface) corrosion. Because its nickel content improves wettability on tungsten carbide tool tips, the largest use is to braze carbide tool assemblies. Melting range and low fluidity make BAg-3 suitable for forming larger fillets or filling wide clearances. This filler metal contains cadmium, and toxic fumes are formed when it is heated.
BAg-4 brazing filler metal, like BAg-3, is used extensively for carbide tip brazing, but flows less freely than BAg-3. This filler metal does not contain cadmium.
BAg-5 and -6 brazing filler metals are frequently used for brazing in the electrical industry. They are also used, along with BAg-7 and -24, in the dairy and food industries where the use of cadmium-containing filler metals is prohibited. BAg-5 is an excellent filler metal for brazing brass parts (such as in ships piping, band instruments, or lamps. Since BAg-6 has a broad melting range and is not as free flowing as BAg-1 and -2, it is a better filler metal for filling wide joint clearances or forming large fillets.
BAg-7 brazing filler metal, a cadmium-free substitute for BAg-1, is low-melting with good flow and wetting properties. Typical applications include:
(1) Food equipment where cadmium must be avoided
(2) Minimizing stress corrosion cracking in nickel or nickel-base alloys at low brazing temperatures
(3) Improving color match where the site color will blend with the base metal
BAg-8 brazing filler metal is suitable for furnace brazing in a protective atmosphere without the use of a flux, as well as for brazing procedures requiring a flux. It is usually used on copper or copper alloys. When molten, BAg-8 is very fluid and may flow out over the workpiece surfaces during some furnace brazing applications. It can also be used on stainless steel, nickel base alloys and carbon steel, although its wetting action on these metals is slow. Higher brazing temperatures will improve flow and wetting.
BAg-Sa brazing filler metal is used for zinc in a protective atmosphere and is advantageous when brazing precipitation hardening and other stainless steels in the 760 to 870°C (1400 to 1600°F) range. The lithium content serves to promote wetting and to increase the flow of the filler metal on difficult-to-braze metals and alloys. Lithium is particularly helpful on base metals containing minor amounts of titanium and aluminum.
BAg-9 and -10 filler metals are used particularly for joining sterling silver. These filler metals have different brazing temperatures, and so can be used for step brazing of successive joints. The color, after brazing, approximates the color of sterling silver.
BAg- 13 brazing filler metal is used for service temperatures up to 370°C (700°F). Its low zinc content makes it suitable for furnace brazing.
BAg-13a brazing filler metal is similar to BAg-13, except that it contains no zinc, which is advantageous where volatilization is objectionable in furnace brazing.
BAg-18 brazing filler metal is similar to BAg-8 in its applications. Its tin content helps promote wetting on stainless steel, nickel-base alloys, and carbon steel. BAg-18 has a lower liquidus than BAg-8 and is used in step-brazing applications and where fluxless braz- ing is important.
BAg-19 brazing filler metal is used for the same applications as BAg-Sa. BAg-19 is often used in higher brazing temperature applications where the precipitation hardening heat treatment and brazing are combined.
BAg-20 brazing filler metal possesses good wetting and flow characteristics and has a brazing temperature range higher than the popular Ag-Cu-Zn-Cd compositions. Due to its good brazing properties, freedom from cadmium, and a more economical silver content, new uses for this filler metal are being developed.
BAg-2 1 brazing filler metal is used in brazing AISU 300 and 400 series stainless steels, as well as the precipitation hardening nickel and steel alloys. BAg-2 1 is particularly suited to protective atmosphere furnace brazing because of the absence of zinc and1 cadmium. It does not require a flux for proper brazing, unless the temperatures are low. It requires a rather hilgh brazing temperature, and it flows in a sluggish manner. The nickel content makes it immune to crevice corrosion, particularly on the 400 series stainless steels, by imparting a nickel-rich layer along the fillet edge. It has been used for brazing stainless steel vanes of gas turbine aircraft engines.
BAg-22 is a low-temperature, cadmium-free filler metal with improved strength characteristics over B Ag-3, particularly in brazing tungsten carbide tools.
BAg-23 is a high-temperature, free-flowing filler metal usable both for torch and protective atmosphere furnace brazing. This filler metal is mainly used in brazing stainless steel, nickel-base and cobalt-base alloys for high temperature applications. If this filler metal is used in a high-vacuum atmosphere, a loss of manganese will occur due to its high vapor pressure. Thus a partial pressure vacuum is desirable.
BAg-24 brazing filler metal is low-melting, free- flowing, cadmium-free, and suitable for use in joining low carbon 300 series stainless steels (particularly food handling equipment and hospital utensils), and small tungsten carbide inserts for cutting tools.
BAg-26 brazing filler metal is a low-silver cadmium-free material suitable for carbide and stainless steel brazing. The low brazing temperature and good flow characteristics make it well suited for moderate strength applications.
BAg-27 brazing filler metal is similar to BAg-2, but has lower silver and is somewhat more subject to liquation due to a wider melting range. This filler metal contains cadmium. Toxic fumes are formed on heating.
BAg-28 brazing filler metal has a lower brazing temperature with a relatively narrower melting range than other cadmium-free classifications with similar silver content. BAg-28 also has free-flowing characteristics.
BAg-33 brazing filler metal was developed to minimize brazing temperature for a filler metal containing 25% silver. It has a lower liquidus and, therefore, a narrower melting range than BAg-27. Its higher total zinc plus cadmium content may require more care during brazing.
BAg-34 brazing filler metal is a cadmium-free material with free-flowing characteristics. The brazing temperature range is similar to that of BAg-2 and BAg-2a, making it an ideal substitute for these filler metals.
The silver copper eutectic (BAg-8), which contains 72% silver and 28% copper, melts at 780°C (1435°F) and is used when zinc in the alloy would give trouble. Alloys containing silver, copper, manganese, and those with a further addition of nickel and silicon are used for similar purposes. Zinc or zinc and cadmium combined with relatively high percentages of silver provide a series of alloys that melt at temperatures between 700 and 760°C (1300 and 1400″F), have a white color, and are used in applications where copper would be objectionable. An alloy containing silver, copper, zinc and cadmium (BAg-la), which flows freely at 635°C (1175°F) is used extensively for joining both ferrous and non-ferrous metals and alloys, because it makes strong joints.
Conductivity- Silver brazing alloys have a higher electrical conductivity than base metal brazing alloys, and therefore their use is particularly desirable for brazing parts of electrical apparatus where the highest conductivity is required. Zinc tends to lower the conductivity, and the silver copper eutectic previously mentioned has about 70% of the conductivity of copper.
Corrosion- Any of the standard silver brazing alloys are resistant to most of the common types of corrosion. When unusual conditions have to be met, it is desirable to make up specimens and subject them to the actual conditions of use in order to determine the best alloy. Galvanic corrosion is a problem, but since it is generally in proportion to the areas exposed to attack, a cathodic joining alloy would give the best result. Silver alloys with high percentages of silver are cathodic to many metals and alloys used to resist corrosive conditions, therefore they are satisfactory for use under such conditions. For example, these high-grade silver alloys are cathodic to nickel-copper alloys and stainless steel under many corrosive conditions for which these metals are used. They should not be used, however, for joining stainless steel when the joints are likely to be attacked by nitric acid.
The question of color match with different metals and alloys is often raised. Those silver brazing alloys with low percentages of silver are yellow and the color becomes whiter as the silver is increased. Alloys with high silver and without any copper are the whitest, but in a properly fitted joint, the band of brazing alloy which is visible is so narrow that any slight difference in color is generally a negligible factor.
Fitting, Cleaning, and Assembling
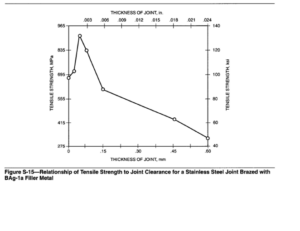
Silver brazing alloys flow freely into narrow openings, and clearances in the range of 0.05 to 0.10 mm (0.002 to 0.004 in.) should be maintained to produce the strongest joints. Figure S-15illustrates the effect of joint clearance on strength. The surfaces of the joint should be clean, and free from all grease, dirt and oxide scale. Any film that prevents the wetting of the joint surfaces will keep a strong bond from being made. After all contaminants have been removed, the surface can be cleaned with emery cloth, washed with an appropriate cleaning solution, or pickled with a suitable solution to remove any scale or highly polished surface that has resulted from rolling or drawing.
A slight roughening of highly polished surfaces by either mechanical or chemical means will assist in good bonding.
When joining flat members, either with lap or butt joints, it is desirable to grind or machine the surfaces of the joint so that they may be held parallel and equi-distant to each other. If thin sheet inserts are used, the parts should be clamped together with enough pressure to hold them firmly together after the alloy has melted.
After the members have been properly cleaned and fitted, the joint surfaces should be protected with a film of flux. This flux must be fluid and chemically active at the melting point of the brazing alloy and should be spread over the entire surface. It is also advisable to protect the brazing alloy with flux when it is fed into the joint.
Borax, or combinations of borax and boric acid are used, but specially prepared fluxes that are fluid and active at lower temperatures are available, and are preferred for the lower melting point alloys. These proprietary fluxes are composed of chemicals that dissolve refractory oxides readily, and should be used when brazing stainless steels.
Furnace Brazing- Furnace brazing is extensively used with silver-base filler metals. Either continuous or batch furnaces are used, and the heatiqg may be supplied by gas or electricity. The atmospllere in the furnace is controlled to prevent oxidation by the use of various types of reducing or non-oxidizing gases. A considerable amount of brazing is done with induction and resistance heating.
Dip Brazing- Dip brazing is another successful method of brazing. The metal bath form of dip brazing is principally used for dipping small parts like terminal wires. Salt bath brazing has been applied to different types of assemblies where the silver brazing alloy can be pre-placed, and the component parts jigged in a satisfactory manner.
Gas Brazing- Gas brazing includes all combinations of torch brazing, such as oxyacetylene, oxyhydrogen, oxygen and natural gas, and butane or propane; also air with these fuel gases. The air-gas and air-acetylene torches will produce satisfactory results with small parts, and the large torches or those with multiple flames may be used on fairly large workpieces.
In order to obtain the full benefit from these low-temperature silver brazing filler metals, the brazer should be trained to observe the rate at which different metals become heated to the brazing temperature, and to give particular attention to the relative mass of each of the members being brazed. Metals of high heat conductivity, such as copper, should be preheated some distance from the joint. If there is much difference in the size of the parts, then the one with largest cross section should be given the most heat.
Even though silver-base brazing filler metals are more expensive than soft solder, they are used for two reasons: (1) the demand of industry for better and quicker methods of joining and fabricating articles and equipment from sheet metal and tubing, and (2) the comparatively low melting points of these alloys, their free-flowing properties and the strength of joints made with them.
Applications
Electrical- Transformer leads and taps are brazed with silver alloys because of the low temperatures at which strong, shock-resistant joints of high conductivity can be made. Joints in bus bar installations of all kinds are made with these alloys because of the high strength, corrosion resistance, and elimination of voltage drop. Ground connections and cable joints are also made with this process.
In the manufacture of electric motors, end rings are bonded to rotor bars; and many small parts in the manufacture of electrical equipment are brazed with silver alloys. Lacing wires and shrouding are joined to turbine blades, and in certain types of turbines, the blades are silver-alloy brazed to packing pieces.
Refrigerators and Air Conditioning- One of the largest uses of silver brazing alloys is in the manufacture of refrigeration units, for both household and industrial plants. The low temperature at which they melt and the strong corrosion-resistant joints make them particularly desirable for joining the light metal sheets and tubing which are used in this industry.
Piping- Standard pipe and fittings up to 25 cm (10 in.) or more in diameter are joined with these alloys, and tests on joints show no failure in the pipe or fitting when the work is done properly. Special fittings are being made with rings of silver brazing alloy fitted into grooves cut in the fittings, and this type of joint has been specified for marine and navy piping, and piping in buildings.
Other Uses– Articles for home, such as cooking utensils, hot water tanks, water heaters, and metal furniture are brazed. Industrial equipment such as chemical equipment and containers, dairy equipment, and innumerable products in the electrical, automotive and aerospace industries are brazed with silver-base metal fillers. See also BRAZING, FURNACES, INDUCTION HEATING, and SALT BATH.
